Eurofins Biomnis
Leader européen du secteur de la biologie médicale spécialisée
Découvrir eurofins biomnisEurofins Biomnis
Moteur de l’innovation en biologie médicale spécialisée
Aide au diagnostic
Tous les contenus
avril 2024
Actualités sur les mycoplasmes urogénitaux et les infections sexuellement transmissibles à Mycoplasma genitalium
Actualités sur les mycoplasmes urogénitaux et les infections sexuellement transmissibles à Mycoplasma genitalium Les mycoplasmes urogénitaux sont des commensaux des voies urogénitales et parfois, des pathogènes opportunistes. Mais seul Mycoplasma genitalium est un agent d’infection…

avril 2024
Mycoplasma genitalium : Ce qu’il faut retenir
Décision du 2 janvier 2024 de l’Union nationale des caisses d’assurance maladie relative à la liste des actes et prestations pris en charge par l’assurance maladie – Légifrance (legifrance.gouv.fr) U. urealyticum (U. parvum) et M….
Actualités
Tous les évènements
Inscrivez-vous dès maintenant pour ne manquer aucune invitation !
Je m'inscris
Textes réglementaires
Journal Officiel n°0176 du 1er août 2023
Parole expert
La prise en charge biologique des hémopathies malignes
Retrouvez nos documents et plaquettes d'infos
Retrouvez les documents (bon de commande, fiche de renseignements cliniques ...) mis à votre disposition pour une bonne prise en charge des analyses demandées

Le séquençage de l’exome dans l’insuffisance ovarienne prématurée : Pour une orientation plus précise et personnalisée des patientes et couples (DS111)

Calendrier 2024 - Dépistage sérique de la Trisomie 21 foetale au 1er ou 2ème trimestre de la grossesse (D21-2024)

Le séquençage de l’exome en contexte prénatal (DS108)
Pour un diagnostic optimisé en cas de malformations foetales

Oncogénétique constitutionnelle : Utilisation du séquençage Long-ReadNanopore - l’exemple du gène PMS2 (DS112)

B59 - Bon de demande : Génétique des pancréatites chroniques et héréditaires

Oncogénétique - Prédispositions héréditaires aux cancers : Intérêt du séquençage de l'exome (DS94)

Dépistage de la trisomie 21, 18 et 13 par analyse de l’ADN libre circulant (ADNlc) (DS29-HP)
Le test de dépistage prénatal sur simple prise de sang maternel


Eurofins Biomnis - Dossier de presse 2021
Espace Presse
Tous les communiqués de pressePrésence internationale
- Implantations Eurofins Biomnis
- Eurofins Biomnis Ireland & UK
- Correspondants Eurofins Biomnis
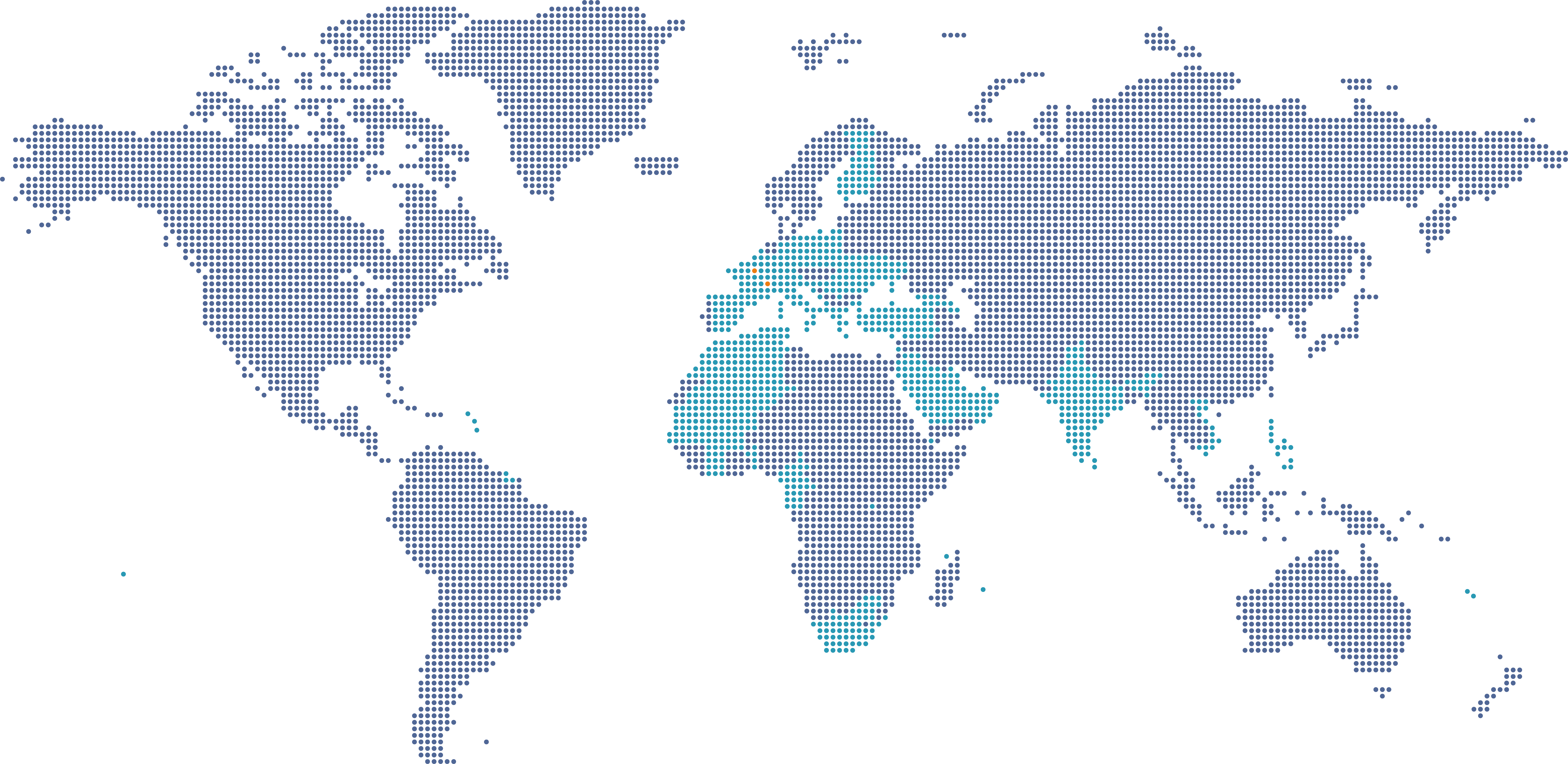
Eurofins Biomnis est présent dans 43 pays
Eurofins Biomnis est présent dans 43 pays et 3 continents. Nous sommes à votre écoute pour vous accompagner dans vos projets.
Contactez-nous- Allergologie
- AMP – Embryologie – Spermiologie
- Biochimie
- Biologie préventive
- Chimie analytique
- Cytogénétique constitutionnelle
- Dépistage T21 – DPNI – Pré-éclampsie – Biochimie fœtale
- Génétique
- Hématologie
- Immunologie
- Infectiologie
- Oncologie solide et hémopathies malignes
- Recherche clinique
- Anatomie et Cytologie Pathologique